Author response:
The following is the authors’ response to the original reviews
Public Reviews:
Reviewer #1 (Public review):
Summary:
In this manuscript, Chengjian Zhao et al. focused on the interactions between vascular, biliary, and neural networks in the liver microenvironment, addressing the critical bottleneck that the lack of high-resolution 3D visualization has hindered understanding of these interactions in liver disease.
Strengths:
This study developed a high-resolution multiplex 3D imaging method that integrates multicolor metallic compound nanoparticle (MCNP) perfusion with optimized CUBIC tissue clearing. This method enables the simultaneous 3D visualization of spatial networks of the portal vein, hepatic artery, bile ducts, and central vein in the mouse liver. The authors reported a perivascular structure termed the Periportal Lamellar Complex (PLC), which is identified along the portal vein axis. This study clarifies that the PLC comprises CD34⁺Sca-1⁺ dual-positive endothelial cells with a distinct gene expression profile, and reveals its colocalization with terminal bile duct branches and sympathetic nerve fibers under physiological conditions.<br />
Weaknesses:
This manuscript is well-written, organized, and informative. However, there are some points that need to be clarified.
(1) After MCNP-dye injection, does it remain in the blood vessels, adsorb onto the cell surface, or permeate into the cells? Does the MCNP-dye have cell selectivity?
The experimental results showed that after injection, the MCNP series nanoparticles predominantly remained within the lumens of blood vessels and bile ducts, with their tissue distribution determined by physical perfusion. No diffusion of the dye signal into the surrounding parenchymal tissue was observed, nor was there any evidence of adsorption onto the cell surface or entry into cells. The newly added Supplementary Figure S2A–H further confirmed this feature, demonstrating that the dye signals were strictly confined to the luminal space, clearly delineating the continuous course of blood vessels and the branching morphology of bile ducts. These findings strongly support the conclusion that “MCNP dyes are distributed exclusively within the luminal compartments.”
Therefore, the MCNP dyes primarily serve as intraluminal tracers within the tissue rather than as labels for specific cell types.
(2) All MCNP-dyes were injected after the mice were sacrificed, and the mice's livers were fixed with PFA. After the blood flow had ceased, how did the authors ensure that the MCNP-dyes were fully and uniformly perfused into the microcirculation of the liver?
Thank you for the reviewer’s valuable comments. Indeed, since all MCNP dyes were perfused after the mice were euthanized and blood circulation had ceased, we cannot fully ensure a homogeneous distribution of the dye within the hepatic microcirculation. The vascular labeling technique based on metallic nanoparticle dyes used in this study offers clear imaging, stable fluorescence intensity, and multiplexing advantages; however, it also has certain limitations. The main issue is that the dye distribution within the hepatic parenchyma can be affected by factors such as lobular overlap, local tissue compression, and variations in vascular pathways, resulting in regional inhomogeneity of dye perfusion. This is particularly evident in areas where multiple lobes converge or where anatomical structures are complex, leading to local dye accumulation or over-perfusion.
In our experiments, we attempted to minimize local blockage or over-perfusion by performing PBS pre-flushing and low-pressure, constant-speed perfusion. Nevertheless, localized dye accumulation or uneven distribution may still occur in lobe junctions or structurally complex regions. Such variation represents one of the methodological limitations. Overall, the dye signals in most samples remained confined to the vascular and biliary lumens, and the distribution pattern was highly reproducible.
We have addressed this issue in the Discussion section but would like to emphasize here that, although this system has clear advantages, it remains sensitive to anatomical variability in the liver—such as lobular overlap and vascular heterogeneity. At vascular junctions, local perfusion inhomogeneity or dye accumulation may occur; therefore, injection strategies and perfusion parameters should be adjusted according to liver size and vascular condition to improve reproducibility and imaging quality. It should also be noted that the results obtained using this method primarily aim to visualize the overall and fine anatomical structures of the hepatic vascular system rather than to quantitatively reflect hemodynamic processes. In the future, we plan to combine in vivo perfusion or dynamic fluid modeling to further validate the diffusion characteristics of the dyes within the hepatic microcirculation.
(3) It is advisable to present additional 3D perspective views in the article, as the current images exhibit very weak 3D effects. Furthermore, it would be better to supplement with some videos to demonstrate the 3D effects of the stained blood vessels.
Thank you for the reviewer’s valuable comments. In response to the suggestion, we have added perspective-rendered images generated from the 3D staining datasets to provide a more intuitive visualization of the spatial morphology of the hepatic vasculature. These images have been included in Figure S2A–J. In addition, we have prepared supplementary videos (available upon request) that dynamically display the three-dimensional distribution of the stained vessels, further enhancing the spatial perception and visualization of the results.
(4) In Figure 1-I, the authors used MCNP-Black to stain the central veins; however, in addition to black, there are also yellow and red stains in the image. The authors need to explain what these stains are in the legend.
Thank you for the reviewer’s constructive comment. In Figure 1I, MCNP-Black labels the central vein (black), MCNP-Yellow labels the portal vein (yellow), MCNP-Pink labels the hepatic artery (pink), and MCNP-Green labels the bile duct (green). We have revised the Figure 1 legend to include detailed descriptions of the color signals and their corresponding structures to avoid any potential confusion.
(5) There is a typo in the title of Figure 4F; it should be "stem cell".
Thank you for the reviewer’s careful correction. We have corrected the spelling error in the title of Figure 4F to “stem cell” and updated it in the revised manuscript.
(6) Nuclear staining is necessary in immunofluorescence staining, especially for Figure 5e. This will help readers distinguish whether the green color in the image corresponds to cells or dye deposits.
We thank the reviewer for the valuable suggestion. We understand that nuclear staining can help determine the origin of fluorescence signals. However, in our three-dimensional imaging system, the deep signal acquisition range after tissue clearing often causes nuclear dyes such as DAPI to generate highly dense and widespread fluorescence, especially in regions rich in vascular structures, which can obscure the fine vascular and perivascular details of interest. Therefore, this study primarily focuses on high-resolution visualization of the spatial architecture of the vascular and biliary systems. We have added an explanation regarding this point in Figures S2I–J.
Reviewer #2 (Public review):
Summary:
The present manuscript of Xu et al. reports a novel clearing and imaging method focusing on the liver. The authors simultaneously visualized the portal vein, hepatic artery, central vein, and bile duct systems by injecting metal compound nanoparticles (MCNPs) with different colors into the portal vein, heart left ventricle, inferior vena cava, and the extrahepatic bile duct, respectively. The method involves: trans-cardiac perfusion with 4% PFA, the injection of MCNPs with different colors, clearing with the modified CUBIC method, cutting 200 micrometer thick slices by vibratome, and then microscopic imaging. The authors also perform various immunostaining (DAB or TSA signal amplification methods) on the tissue slices from MCNP-perfused tissue blocks. With the application of this methodical approach, the authors report dense and very fine vascular branches along the portal vein. The authors name them as 'periportal lamellar complex (PLC)' and report that PLC fine branches are directly connected to the sinusoids. The authors also claim that these structures co-localize with terminal bile duct branches and sympathetic nerve fibers, and contain endothelial cells with a distinct gene expression profile. Finally, the authors claim that PLC-s proliferate in liver fibrosis (CCl4 model) and act as a scaffold for proliferating bile ducts in ductular reaction and for ectopic parenchymal sympathetic nerve sprouting.
Strengths:
The simultaneous visualization of different hepatic vascular compartments and their combination with immunostaining is a potentially interesting novel methodological approach.
Weaknesses:
This reviewer has several concerns about the validity of the microscopic/morphological findings as well as the transcriptomics results. In this reviewer's opinion, the introduction contains overstatements regarding the potential of the method, there are severe caveats in the method descriptions, and several parts of the Results are not fully supported by the documentation. Thus, the conclusions of the paper may be critically viewed in their present form and may need reconsideration by the authors.
We sincerely thank the reviewer for the thorough evaluation and constructive comments on our study. We fully understand and appreciate the reviewer’s concerns regarding the methodological validity and interpretation of the results. In response, we have made comprehensive revisions and additions to the manuscript as follows:
First, we have carefully revised the Introduction and Discussion sections to provide a more balanced description of the methodological potential, removing statements that might be considered overstated, and clarifying the applicable scope and limitations of our approach (see the revised Introduction and Discussion).
Second, we have substantially expanded the Methods section with detailed information on model construction, imaging parameters, data processing workflow, and technical aspects of the single-cell transcriptomic reanalysis, to enhance the transparency and reproducibility of the study.
Third, we have added additional references and explanatory notes in the Results section to better support the main conclusions (see Section 6 of the Results).
Finally, we have rechecked and validated all experimental data, and conducted a verification analysis using an independent single-cell RNA-seq dataset (Figure S6). The results confirm that the morphological observations and transcriptomic findings are consistent and reproducible across independent experiments.
We believe these revisions have greatly strengthened the reliability of our conclusions and the overall scientific rigor of the manuscript. Once again, we sincerely appreciate the reviewer’s valuable comments, which have been very helpful in improving the logic and clarity of our work.
Reviewer #3 (Public review):
Summary:
In the reviewed manuscript, researchers aimed to overcome the obstacles of high-resolution imaging of intact liver tissue. They report successful modification of the existing CUBIC protocol into Liver-CUBIC, a high-resolution multiplex 3D imaging method that integrates multicolor metallic compound nanoparticle (MCNP) perfusion with optimized liver tissue clearing, significantly reducing clearing time and enabling simultaneous 3D visualization of the portal vein, hepatic artery, bile ducts, and central vein spatial networks in the mouse liver. Using this novel platform, the researchers describe a previously unrecognized perivascular structure they termed Periportal Lamellar Complex (PLC), regularly distributed along the portal vein axis. The PLC originates from the portal vein and is characterized by a unique population of CD34⁺Sca-1⁺ dual-positive endothelial cells. Using available scRNAseq data, the authors assessed the CD34⁺Sca-1⁺ cells' expression profile, highlighting the mRNA presence of genes linked to neurodevelopment, biliary function, and hematopoietic niche potential. Different aspects of this analysis were then addressed by protein staining of selected marker proteins in the mouse liver tissue. Next, the authors addressed how the PLC and biliary system react to CCL4-induced liver fibrosis, implying PLC dynamically extends, acting as a scaffold that guides the migration and expansion of terminal bile ducts and sympathetic nerve fibers into the hepatic parenchyma upon injury.
The work clearly demonstrates the usefulness of the Liver-CUBIC technique and the improvement of both resolution and complexity of the information, gained by simultaneous visualization of multiple vascular and biliary systems of the liver at the same time. The identification of PLC and the interpretation of its function represent an intriguing set of observations that will surely attract the attention of liver biologists as well as hepatologists; however, some claims need more thorough assessment by functional experimental approaches to decipher the functional molecules and the sequence of events before establishing the PLC as the key hub governing the activity of biliary, arterial, and neuronal liver systems. Similarly, the level of detail of the methods section does not appear to be sufficient to exactly recapitulate the performed experiments, which is of concern, given that the new technique is a cornerstone of the manuscript.
Nevertheless, the work does bring a clear new insight into the liver structure and functional units and greatly improves the methodological toolbox to study it even further, and thus fully deserves the attention of readers.
Strengths:
The authors clearly demonstrate an improved technique tailored to the visualization of the liver vasulo-biliary architecture in unprecedented resolution.
This work proposes a new biological framework between the portal vein, hepatic arteries, biliary tree, and intrahepatic innervation, centered at previously underappreciated protrusions of the portal veins - the Periportal Lamellar Complexes (PLCs).
Weaknesses:
Possible overinterpretation of the CD34+Sca1+ findings was built on re-analysis of one scRNAseq dataset.
Lack of detail in the materials and methods section greatly limits the usefulness of the new technique to other researchers.
We thank the reviewer for this important comment. We agree that when conclusions are mainly based on a single dataset, overinterpretation should be avoided. In response to this concern, we have carefully re-evaluated and clearly limited the scope of our interpretation of the scRNA-seq analysis. In addition, we performed a validation analysis using an independent single-cell RNA-seq dataset (see new Figure S6), which consistently confirmed the presence and characteristic transcriptional profile of the periportal CD34⁺Sca1⁺ endothelial cell population. These supplementary analyses strengthen the robustness of our findings and address the reviewer’s concern regarding potential overinterpretation.
In the revised manuscript, we have also greatly expanded the Materials and Methods section by providing detailed information on sample preparation, imaging parameters, data processing workflow, and single-cell reanalysis procedures. These revisions substantially improve the transparency and reproducibility of our methodology, thereby enhancing the usability and reference value of this technique for other researchers.
Recommendations for the authors:
Reviewer #2 (Recommendations for the authors):
Introduction
(1) In general, the Introduction is very lengthy and repetitive. It needs extensive shortening to a maximum of 2 A4 pages.
We thank the reviewer for the valuable suggestions. We have thoroughly condensed and restructured the Introduction, removing redundant content and merging related paragraphs to make the theme more focused and the logic clearer. The revised Introduction has been shortened to within two A4 pages, emphasizing the scientific question, innovation, and technical approach of the study.
(2) Please correct this erroneous sentence:
'...the liver has evolved the most complex and densely n organized vascular network in the body, consisting primarily of the portal vein system, central vein system, hepatic artery system, biliary system, and intrahepatic autonomic nerve network [6, 7].'
We thank the reviewer for pointing out this spelling error. The revised sentence is as follows:
“…the liver has evolved the most complex and densely organized ductal-vascular network in the body, consisting primarily of the portal vein system, central vein system, hepatic artery system, biliary system, and intrahepatic autonomic nerve network [6, 7].”
(3) '...we achieved a 63.89% improvement in clearing efficiency and a 20.12% increase in tissue transparency'
Please clarify what you exactly mean by 'clearing efficiency' and 'increased tissue transparency'.
We thank the reviewer for the valuable comments and have clarified the relevant terminology in the revised manuscript.
“Clearing efficiency” refers to the improvement in the time required for the liver tissue to become completely transparent when treated with the optimized Liver-CUBIC protocol (40% urea + H₂O₂), compared with the conventional CUBIC method. In this study, the clearing time was reduced from 9 days to 3.25 days, representing a 63.89% increase in time efficiency.
“Tissue transparency” refers to the ability of the cleared tissue to transmit visible light. We quantified the optical transparency by measuring light transmittance across the 400–900 nm wavelength range using a microplate reader. The results showed that the average transmittance increased by 20.12%, indicating that Liver-CUBIC treatment markedly enhanced the optical clarity of the liver tissue.
(4) I am concerned about claiming this imaging method as real '3D imaging'. Namely, while the authors clear full lobes, they actually cut the cleared lobes into 200-micrometer-thick slices and perform further microscopy imaging on these slices. Considering that they focus on ductular structures of the liver (such as vasculature, bile duct system, and innervations), 200 micrometer allows a very limited 3D overview, particularly in comparison with the whole-mount immuno-imaging methods combined with light sheet microscopy (such as Adori 2021, Liu 2021, etc). In this context, I feel several parts of the Introduction to be an overstatement: besides of emphasizing the advantages of the technique (such as simultaneous visualization of different hepatic vascular compartments and the bile duct system by MCNPs, the combination with immunostainings), the authors must honestly discuss the limitations (such as limited tissue overview, potential dye perfusion problems - uneven distribution of the dye etc).
We appreciate the reviewer’s insightful comments. It is true that most of the imaging depth in this study was limited to approximately 200 μm, and thus it could not achieve whole-liver three-dimensional imaging comparable to light-sheet microscopy. However, the primary focus of our study was to resolve the microscopic intrahepatic architecture, particularly the spatial relationships among blood vessels, bile ducts, and nerve fibers. Through high-resolution imaging of thick tissue sections, combined with MCNP-based multichannel labeling and immunofluorescence co-staining, we were able to accurately delineate the three-dimensional distribution of these microstructures within localized regions.
In addition to thick-section imaging, we also obtained whole-lobe dye perfusion data (as shown in Figure S1F), which comprehensively depict the three-dimensional branching patterns and distribution of the vascular systems within the liver lobe. These images were acquired from intact liver lobes perfused with MCNP dyes, revealing a continuous vascular network extending from major trunks to peripheral branches, thereby demonstrating that our approach is also capable of achieving organ-level visualization.
We have added this image and a corresponding description in the revised manuscript to more comprehensively present the coverage of our imaging system, and we have incorporated this clarification into the Discussion section.
Method
(5) More information may be needed about MCNPs:
a) As reported, there are nanoparticles with different colors in brightfield microscopy, but the particles are also excitable in fluorescence microscopy. Would you please provide a summary about excitation/emission wavelengths of the different MCNPs? This is crucial to understand to what extent the method is compatible with fluorescence immunohistochemistry.
We thank the reviewer for the careful attention and professional suggestion. We fully agree that this issue is critical for evaluating the compatibility of our method with fluorescent immunohistochemistry. Different types of metal compound nanoparticles (MCNPs) have clearly distinguishable spectral properties:
- MCNP-Green and MCNP-Yellow: AF488-matched spectra, with excitation/emission wavelengths of 495/519 nm.
- MCNP-Pink: Designed for far-red spectra, with excitation/emission wavelengths of 561/640 nm.
- MCNP-Black: Non-fluorescent, appearing black under bright-field microscopy only.
The above information has been added to the Materials and Methods section.
b) Also, is there more systematic information available concerning the advantage of these particles compared to 'traditional' fluorescence dyes, such as Alexa fluor or Cy-dyes, in fluorescence microscopy and concerning their compatibility with various tissue clearing methods (e.g., with the frequently used organic-solvent-based methods)?
We thank the reviewer for the detailed question. Compared with conventional organic fluorescent dyes, MCNP offers the following advantages:
- Enhanced photostability: Its inorganic core-shell structure resists fading even after hydrogen peroxide bleaching.
- High signal stability: Fluorescence is maintained during aqueous-based clearing (e.g., CUBIC) and multiple rounds of staining without quenching.
We appreciate the reviewer’s suggestion. In our Liver-CUBIC system, MCNP nanoparticles exhibited excellent multi-channel labeling stability and fluorescence signal retention. Regarding compatibility with other clearing methods (e.g., SCAFE, SeeDB, CUBIC), since these methods have limited effectiveness for whole-liver clearing (see Figure 2 of Tainaka, et al. 2014) and cannot meet the requirements for high-resolution microstructural imaging in this study, we consider further testing of their compatibility unnecessary.
In summary, MCNP dye demonstrates superior signal stability and spectral separation compared with conventional organic fluorescent dyes in multi-channel, long-term, high-transparency three-dimensional tissue imaging.
c) When you perfuse these particles, to which structures do they bind inside the ducts (vessels, bile ducts)? Is the 48h post-fixation enough to keep them inside the tubes/bind them to the vessel walls? Is there any 'wash-out' during the complex cutting/staining procedure? E.g., in Figure 2D: the 'classical' hepatic artery in the portal triad is not visible - but the MCNP apparently penetrated to the adjacent sinusoids at the edge of the lobulus. Also, in Figure 3B, there is a significant mismatch between the MNCP-green (bile duct) signal and the CD19 (epithelium marker) immunostaining. Please discuss these.
The experimental results showed that following injection, MCNP nanoparticles primarily remained within the vascular and biliary lumens, and their tissue distribution depended on physical perfusion. No dye signal was observed to diffuse into the surrounding parenchyma, nor did the particles adhere to cell surfaces or enter cells. The newly added Supplementary Figures S2A–H further confirm this feature: the dye signal is strictly confined within the lumens, clearly delineating continuous vascular paths and biliary branching patterns, strongly supporting the conclusion that “MCNP dye is distributed only within luminal spaces.”
Thus, MCNP dye mainly serves as an intraluminal tracer rather than a label for specific cell types.
We provide the following explanations and analyses regarding MCNP distribution in the hepatic vascular and biliary systems and its post-fixation stability:
- Potential signal displacement during sectioning/immunostaining: During slicing and immunostaining, a small number of particles may be washed away due to mechanical cutting or washing steps; however, the overall three-dimensional structure retains high spatial fidelity.
- Observation in Figure 2D: MCNP was seen entering the sinusoidal spaces at the lobule periphery, but hepatic arteries were not visible, likely due to limitations in section thickness. Although arteries were not apparent in this slice, arterial distribution around the portal vein is visible in Figure 2C. It should be noted that Figures 2C, D, and E do not represent whole-liver imaging, so not all regions necessarily contain visible hepatic arteries. For easier identification, the main hepatic artery trunk is highlighted in cyan in Figure 2E.
- Incomplete biliary signal in Figure 3B: This may be because CK19 labeling only covers biliary epithelial cells, whereas MCNP-green distributes throughout the biliary lumen. In Figure 3B, the terminal MCNP-green signal exhibits irregular polygonal structures, which we interpret as the canalicular regions.
(6) Which fixative was used for 48h of postfixation (step 6) after MCNP injections?
After MCNP injection, mouse livers were post-fixed in 4% paraformaldehyde (PFA) for 48 hours. This fixation condition effectively “locks” the MCNP particles within the vascular and biliary lumens, maintaining their spatial positions, while also being compatible with subsequent sectioning and multi-channel immunostaining analyses.
The above information has been added to the Materials and Methods section
(7) What is the 'desired thickness' in step 7? In the case of immunostained tissue, a 200-micrometer slice thickness is mentioned. However, based on the Methods, it is not completely clear what the actual thickness of the tissue was that was examined ultimately in the microscopes, and whether or not the clearing preceded the cutting or vice versa.
We appreciate the reviewer’s question. The “desired thickness” referred to in step 7 of the manuscript corresponds to the thickness of tissue sections used for immunostaining and high-resolution microscopic imaging, which is typically around 200 µm. We selected 200 µm because this thickness is sufficient to observe the PLC structure in its entirety, allows efficient staining, and preserves tissue architecture well. Other researchers may choose different section thicknesses according to their experimental needs.
In this study, the processing order for immunostained tissue samples was sectioning followed by clearing, as detailed below:
Section Thickness
To ensure antibody penetration and preservation of three-dimensional structure, tissue sections were typically cut to ~200 µm. Thicker sections can be used if more complete three-dimensional structures are required, but adjustments may be needed based on antibody penetration and fluorescence detection conditions.
Clearing Sequence
After sectioning, slices were processed using the Liver-CUBIC aqueous-based clearing system.
(8) More information is needed concerning the 'deep-focus microscopy' (Keyence), the applied confocal system, and the THUNDER 'high resolution imaging system': basic technical information, resolutions, objectives (N.A., working distance), lasers/illumination, filters, etc.
In this study, all liver lobes (left, right, caudate, and quadrate lobes) were subjected to Liver-CUBIC aqueous-based clearing to ensure uniform visualization of MCNP fluorescence and immunolabeling throughout the three-dimensional imaging of the entire liver.
The above information has been added to the Materials and Methods section.
Imaging Systems and Settings
VHX-6000 Extended Depth-of-Field Microscope: Objective: VH-Z100R, 100×–1000×; resolution: 1 µm (typical); illumination: coaxial reflected; transmitted illumination on platform: ON.
Zeiss Confocal Microscope (980): Objectives: 20× or 40×; image size: 1024 × 1024. Fluorescence detection was set up in three channels:
- Channel 1: 639 nm laser, excitation 650 nm, emission 673 nm, detection range 673–758 nm, corresponding to Cy5-T1 (red).
- Channel 2: 561 nm laser, excitation 548 nm, emission 561 nm, detection range 547–637 nm, corresponding to Cy3-T2 (orange).
- Channel 3: 488 nm laser, excitation 493 nm, emission 517 nm, detection range 490–529 nm, corresponding to AF488-T3 (green).
Leica THUNDER Imager 3D Tissue: Fluorescence detection in two channels:
- Channel 1: FITC channel (excitation 488 nm, emission ~520 nm).
- Channel 2: Orange-red channel (excitation/emission 561/640 nm).<br />
Equipped with matching filter sets to ensure signal separation.
The above information has been added to the Materials and Methods section.
(9) Liver-CUBIC, step 2: which lobe(s) did you clear (...whole liver lobes...).
In this study, all liver lobes (left, right, caudate, and quadrate lobes) were subjected to Liver-CUBIC aqueous-based clearing to ensure uniform visualization of MCNP fluorescence and immunolabeling throughout the three-dimensional imaging of the entire liver.
The above information has been added to the Materials and Methods section.
(10) For the DAB and TSA IHC stainings, did you use free-floating slices, or did you mount the vibratome sections and do the staining on mounted sections?
In this study, fixed livers were first sectioned into thick slices (~200 µm) using a vibratome. Subsequently, DAB and TSA immunohistochemical (IHC) staining were performed on free-floating sections. During the entire staining process, the slices were kept floating in the solutions, ensuring thorough antibody penetration in the thick sections while preserving the three-dimensional tissue architecture, thereby facilitating multiple rounds of staining and three-dimensional imaging.
(11) Regarding the 'transmission quantification': this was measured on 1 mm thick slices. While it is interesting to make a comparison between different clearing methods in general, one must note that it is relatively easy to clear 1mm thick tissue slices with almost any kind of clearing technique and in any tissues. The 'real' differences come with thicker blocks, such as >5mm in the thinnest dimension. Do you have such experiences (e.g., comparison in whole 'left lateral liver lobes')?
In this study, we performed three-dimensional visualization of entire liver lobes to depict the distribution of MCNPs and the overall spatial architecture of the vascular and biliary systems (Figure S1F). However, due to the limitations of the plate reader and fluorescence imaging systems in terms of spatial resolution and light penetration depth, quantitative analyses were conducted only on tissue sections approximately 1 mm thick.
Regarding the comparative quantification of different clearing methods, as the reviewer noted, nearly all aqueous- or organic solvent–based clearing techniques can achieve relatively uniform transparency in 1 mm-thick tissue sections, so differences at this thickness are limited. We have not yet conducted systematic comparisons on whole-lobe sections thicker than 5 mm and therefore cannot provide “true” difference data for thicker tissues.
(12) There is no method description for the ELMI studies in the Methods.
Transmission Electron Microscopy (TEM) Analysis of MCNPs
Before imaging, the MCNP dye solution was centrifuged at 14,000 × g for 10 minutes at 4 °C to remove aggregates and impurities. The supernatant was collected, diluted 50-fold, and 3–4 μL of the sample was applied onto freshly glow-discharged Quantifoil R1.2/1.3 copper grids (Electron Microscopy Sciences, 300 mesh). The sample was allowed to sit for 30 seconds to enable particle adsorption, after which excess liquid was gently wicked away with filter paper and the grid was air-dried at room temperature. The sample was then negatively stained with 1% uranyl acetate for 30 seconds and air-dried again before imaging.
Negative-stain TEM images were acquired using a JEOL JEM-1400 transmission electron microscope operating at 120 kV and equipped with a CCD camera. Data acquisition followed standard imaging conditions.
The above information has been added to the Materials and Methods section.
(13) Please, provide a method description for the applied CCl4 cirrhosis model. This is completely missing.
(1) Under a fume hood, carbon tetrachloride (CCl₄) was dissolved in corn oil at a 1:3 volume ratio to prepare a working solution, which was filtered through a 0.2 μm filter into a 30 mL glass vial. In our laboratory, to mimic chronic injury, mice in the experimental group were intraperitoneally injected at a dose of 1 mL/kg body weight per administration.
(2) Mice were carefully removed from the cage and placed on a scale to record body weight for calculation of the injection volume.
(3) The needle cap was carefully removed, and the required volume of the pre-prepared CCl₄ solution was drawn into the syringe. The syringe was gently flicked to remove any air bubbles.
(4) Mice were placed on a textured surface (e.g., wire cage) and restrained. When the mouse was properly positioned, ideally with the head lowered about 30°, the left lower or right lower abdominal quadrant was identified.
(5) Holding the syringe at a 45° angle, with the bevel facing up, the needle was inserted approximately 4–5 mm into the abdominal wall, and the calculated volume of CCl₄ was injected.
(6) Mice were returned to their cage and observed for any signs of discomfort.
(7) Needles and syringes were disposed of in a sharps container without recapping. A new syringe or needle was used for each mouse.
(8) To establish a progressive liver fibrosis model, injections were administered twice per week (e.g., Monday and Thursday) for 3 or 6 consecutive weeks (n=3 per group). Control mice were injected with an equal volume of corn oil for 3 or 6 weeks (n=3 per group).
(9) Forty-eight hours after the last injection, mice were euthanized by cervical dislocation, and livers were rapidly harvested. Portions of the liver were processed for paraffin embedding and histological sectioning, while the remaining tissue was either immediately frozen or used for subsequent molecular biology analyses.
The above information has been added to the Materials and Methods section.
(14) Please provide a method description for the quantifications reported in Figures 5D, 5F, and 6E.
ImageJ software was used to analyze 3D stained images (Figs. 5F, 6E), and the ultra-depth-of-field 3D analysis module was used to analyze 3D DAB images (Fig. 5D). The specific steps are as follows:
Figure 5D: DAB-stained 3D images from the control group and the CCl<sub>4</sub> 6-week (CCl<sub>4</sub>-6W) group were analyzed. For each group, 20 terminal bile duct branch nodes were randomly selected, and the actual path distance along the branch to the nearest portal vein surface was measured. All measurements were plotted as scatter plots to reflect the spatial extension of bile ducts relative to the portal vein under different conditions.
Figure 5F: TSA 3D multiplex-stained images from the control group, CCl<sub>4</sub> 3-week (CCl<sub>4</sub>-3W), and CCl<sub>4</sub> 6-week (CCl<sub>4</sub>-6W) groups were analyzed. For each group, 5 terminal bile duct branch nodes were randomly selected, and the actual path distance along the branch to the nearest portal vein surface was measured. Measurements were plotted as scatter plots to illustrate bile duct spatial extension.
Figure 6E: TSA 3D multiplex-stained images from the control, CCl<sub>4</sub>-3W, and CCl<sub>4</sub>-6W groups were analyzed. For each group, 5 terminal nerve branch nodes were randomly selected, and the actual path distance along the branch to the nearest portal vein surface was measured. Scatter plots were generated to depict the spatial distribution of nerves under different treatment conditions.
(15) Please provide a method description for the human liver samples you used in Figure S6. Patient data, fixation, etc...
The human liver tissue samples shown in Figure S6 were obtained from adjacent non-tumor liver tissues resected during surgical operations at West China Hospital, Sichuan University. All samples used were anonymized archived tissues, which were applied for scientific research in accordance with institutional ethical guidelines and did not involve any identifiable patient information. After being fixed in 10% neutral formalin for 24 hours, the tissues were routinely processed for paraffin embedding (FFPE), and sectioned into 4 μm-thick slices for immunostaining and fluorescence imaging.
Results
(16) While it is stated in the Methods that certain color MCNPs were used for labelling different structures (i.e., yellow: hepatic artery; green: bile duct; portal vein: pink; central veins: black), in some figures, apparently different color MCNPs are used for the respective structures. E.g., in Figure 1J, the artery is pink and the portal vein is green. Please clarify this.
The color assignment of MCNP dyes is not fixed across different experiments or schematic illustrations. MCNP dyes of different colors are fundamentally identical in their physical and chemical properties and do not exhibit specific binding or affinity for particular vascular structures. We select different colors based on experimental design and imaging presentation needs to facilitate distinction and visualization, thereby enhancing recognition in 3D reconstruction and image display. Therefore, the color labeling in Figure 1F is primarily intended to illustrate the distribution of different vascular systems, rather than indicating a fixed correspondence to a specific dye or injection color.
(17) In Figure 1J, the hepatic artery is extremely shrunk, while the portal vein is extremely dilated - compared to the physiological situation. Does it relate to the perfusion conditions?
We appreciate the reviewer’s attention. In fact, under normal physiological conditions, the hepatic arteries labeled by CD31 are naturally narrow. Therefore, the relatively thin hepatic arteries and thicker portal veins shown in Figure 1J are normal and unrelated to the perfusion conditions. See figure 1E of Adori et al., 2021.
(18) Re: MCNP-black labelled 'oval fenestrae': the Results state 50-100 nm, while they are apparently 5-10-micron diameter in Figure 1I. Accordingly, the comparison with the ELMI studies in the subsequent paragraph is inappropriate.
We thank the reviewer for the correction. The previous statement was a typographical error. In fact, the diameter of the “elliptical windows” marked by MCNP-black is 5–10 μm, so the diameter of 5–10 μm shown in Figure 1I is correct.
(19) Please, correct this erroneous sentence: 'Pink marked the hepatic arterial system by injection extrahepatic duct (Figure 2B).'
Original sentence: “The hepatic arterial system was labeled in pink by injection through the extrahepatic duct (Figure 2B).”
Revised sentence: “The hepatic arterial system was labeled in pink by injection through the left ventricle (Figure 2B).”
(20) How do you define the 'primary portal vein tract'?
We thank the reviewer for the question. The term “primary portal vein tract” refers to the first-order branches of the portal vein that enter the liver from the hepatic hilum. These are the major branches arising directly from the main portal vein trunk and are responsible for supplying blood to the respective hepatic lobes. This definition corresponds to the concept of the first-order portal vein in hepatic anatomy.
(21) I am concerned that the 'periportal lamellar complex (PLC)' that the Authors describe really exists as a distinct anatomical or functional unit. I also see these in 3D scans - in my opinion, these are fine, lower-order portal vein branches that connect the portal veins to the adjacent sinusoid. The strong MCNP-labelling of these structures may be caused by the 'sticking' of the perfused MCNP solutions in these 'pockets' during the perfusion process. What do these structures look like with SMA or CD31 immunostaining? Also, one may consider that the anatomical evaluation of these structures may have limitations in tissue slices. Have you ever checked MCNP-perfused, cleared full live lobes in light sheet microscope scans? I think this would be very useful to have a comprehensive morphological overview. Unfortunately, based on the presented documentation, I am also not convinced that PLCs are 'co-localize' with fine terminal bile duct branches (Figure 3E, S3C), or with TH+ 'neuronal bead chain networks' (Fig 6C). More detailed and more convincing documentation is needed here.
We thank the reviewer for the detailed comments. Regarding the existence and function of the periportal lamellar complex (PLC), our observations are based on MCNP-Pink labeling of the portal vein, through which we were able to identify the PLC structure surrounding the portal branches. It should be noted that the PLC represents a very small anatomical structure. Although we have not yet performed light-sheet microscopy scanning, we anticipate that such imaging would primarily visualize larger portal vein branches. Nevertheless, this does not affect our overall conclusions.
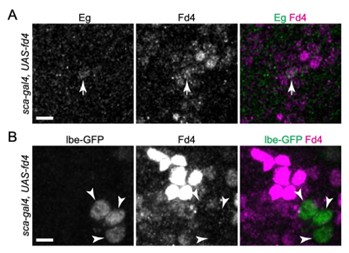
We also appreciate the reviewer’s suggestion that the observed structures might result from MCNP adherence during perfusion. To verify the structural characteristics of the PLC, we performed immunostaining for SMA and CD31, which revealed a specific arrangement pattern of smooth muscle and endothelial markers rather than simple perfusion-induced deposition (Figures 4F and S6B).
Regarding the apparent colocalization of the PLC with terminal bile duct branches (Figures 3E and S3C) and TH⁺ neuronal bead-like networks (Figure 6C), we acknowledge that current literature evidence remains limited. Therefore, we have carefully described these observations as possible spatial associations rather than definitive conclusions. Future studies integrating high-resolution three-dimensional imaging with functional analyses will help to further clarify the anatomical and physiological significance of the PLC.
(22) 'Extended depth-of-field three-dimensional bright-field imaging revealed a strict 1:1 anatomical association between the primary portal vein trunk (diameter 280 {plus minus} 32 μm) and the first-order bile duct (diameter 69 {plus minus} 8 μm) (Figures 3A and S3A)'.
How do you define '1:1 anatomical association'? How do you define and identify the 'order' (primary, secondary) of vessel and bile duct branches in 200-micrometer slices?
We thank the reviewer for the question. In this study, the term “1:1 anatomical correlation” refers to the stable paired spatial relationship between the main portal vein trunk and its corresponding primary bile duct within the same portal territory. In other words, each main portal vein branch is accompanied by a primary bile duct of matching branching order and trajectory, together forming a “vascular–biliary bundle.”
The definitions of “primary” and “secondary” branches were based on extended-depth 3D bright-field reconstructions, considering both branching hierarchy and vessel/duct diameters: primary branches arise directly from the main trunk at the hepatic hilum and exhibit the largest diameters (averaging 280 ± 32 μm for the portal vein and 69 ± 8 μm for the bile duct), whereas secondary branches extend from the primary branches toward the lobular interior with smaller calibers.
(23) In my opinion, the applied methodical approach in the single cell transcriptomics part (data mining in the existing liver single cell database and performing Venn diagram intersection analysis in hepatic endothelial subpopulations) is largely inappropriate and thus, all the statements here are purely speculative. In my opinion, to identify the molecular characteristics of such small and spatially highly organized structures like those fine radial portal branches, the only way is to perform high-resolution spatial transcriptomic.
We thank the reviewer for the comment. We fully acknowledge the importance of high-resolution spatial transcriptomics in identifying the fine structural characteristics of portal vein branches. Due to current funding and technical limitations, we were unable to perform such high-resolution spatial transcriptomic analyses. However, we validated the molecular features of the PLC using another publicly available liver single-cell RNA-sequencing dataset, which provided preliminary supporting evidence (Figures S6B and S6C). In the manuscript, we have carefully stated that this analysis is exploratory in nature and have avoided overinterpretation. In future studies, high-resolution spatial omics approaches will be invaluable for more precisely delineating the molecular characteristics of these fine structures.
(24) 'How the autonomic nervous system regulates liver function in mice despite the apparent absence of substantive nerve fiber invasion into the parenchyma remains unclear.'
Please consider the role of gap junctions between hepatocytes (e.g., Miyashita, 1991; Seseke, 1992).
In this study, we analyzed the spatial distribution of hepatic nerves in mice using immunofluorescence staining and found that nerve fibers were almost exclusively confined to the portal vein region (Figure S6A). Notably, this distribution pattern differs markedly from that in humans. Previous studies have shown that, in human livers, nerves are not only located around the portal veins but also present along the central veins, interlobular septa, and within the parenchymal connective tissue (Miller et al., 2021; Yi, la Fleur, Fliers & Kalsbeek, 2010).
Further research has provided a physiological explanation for this interspecies difference: even among species with distinct sympathetic innervation patterns in the parenchyma—i.e., with or without direct sympathetic input—the sympathetic efferent regulatory functions may remain comparable (Beckh, Fuchs, Ballé & Jungermann, 1990). This is because signals released from aminergic and peptidergic nerve terminals can be transmitted to hepatocytes through gap junctions as electrical signals (Hertzberg & Gilula, 1979; Jensen, Alpini & Glaser, 2013; Seseke, Gardemann & Jungermann, 1992; Taher, Farr & Adeli, 2017).
However, the scarcity of nerve fibers within the mouse hepatic parenchyma suggests that the mechanisms by which the autonomic nervous system regulates liver function in mice may differ from those in humans. This observation prompted us to further investigate the potential role of PLC endothelial cells in this process.
(25) Please, correct typos throughout the text.
We thank the reviewer for this comment. We have carefully proofread the entire manuscript and corrected all typographical errors and minor language issues throughout the text.
Reviewer #3 (Recommendations for the authors):
(1) A strong recommendation - the authors ought to challenge their scRNAsq- re-analysis with another scRNAseq dataset, namely a recently published atlas of adult liver endothelial, but also mesenchymal, immune, and parenchymal cell populations https://pubmed.ncbi.nlm.nih.gov/40954217/, performed with Smart-seq2 approach, which is perfectly suitable as it brings higher resolution data, and extensive cluster identity validation with stainings. Pietilä et al. indicate a clear distinction of portal vein endothelial cells into two populations that express Adgrg6, Jag1 (e2c), from Vegfc double-positive populations (e5c and e2c). Moreover, the dataset also includes the arterial endothelial cells that were shown to be part of the PLC, but were not followed up with the scRNAseq analysis. This distinction could help the authors to further validate their results, better controlling for cross-contaminations that may occur during scRNAseq preparation.
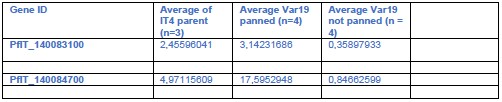
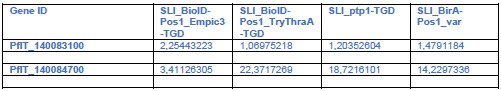
We thank the reviewer for the valuable suggestion. As noted, we have further validated the molecular characteristics of the PLC using a recently published atlas of adult liver endothelial cells (Pietilä et al., 2023, PMID: 40954217). This dataset, generated using the Smart-seq2 technique, provides high-resolution transcriptomic profiles. By analyzing this dataset, we identified a CD34⁺LY6A⁺ portal vein endothelial cell population within the e2 cluster, which is localized around the portal vein. We then examined pathways and gene expression patterns related to hematopoiesis, bile duct formation, and neural signaling within these cells. The results revealed gene enrichment patterns consistent with those observed in our primary dataset, further supporting the robustness of our analysis of the PLC’s molecular characteristics.
(2) Improving the methods section is highly recommended, this includes more detailed information for material and protocols used - catalog numbers; protocol details of the usage - rocking platforms, timing, and tubes used for incubations; GitHub or similar page with code used for the scRNA seq re-analysis.
We thank the reviewer for the valuable suggestion. We have added more detailed information regarding the materials and experimental procedures in the Methods section, including catalog numbers, incubation conditions (such as the type of shaker, incubation time, and tube specifications), and other relevant parameters.
(3) In Figure 2A, the authors claim the size of the nanoparticle is 100nm, while based on the image, the size is ~150-180nm. A more thorough quantification of the particle size would help users estimate the usability of their method for further applications.
We thank the reviewer for the comment. In the TEM image shown in Figure 2A, the nanoparticles indeed appear to be approximately 150–200 nm in size. We have re-verified the particle dimensions and will update the corresponding description in the Methods section to allow readers to more accurately assess the applicability of this approach.
(4) In Figure 3E, it is not clear what is labeled by the pink signal. Please consider labeling the structures in the figure.
We thank the reviewer for the valuable comment. The pink signal in Figure 3E was originally intended to label the hepatic artery. However, a slight spatial misalignment occurred during the labeling process, making its position appear closer to the central vein rather than the portal vein in the image. To avoid misunderstanding, we will add clear annotations to the image and clarify this deviation in the figure legend in the revised version. It should also be noted that this figure primarily aims to illustrate the spatial relationship between the bile duct and the portal vein, and this minor deviation does not affect the reliability of our experimental conclusions.
(5) The following statement is not backed by quantification as it ought to be „Dual-channel three-dimensional confocal imaging combined with CK19 immunostaining revealed that the sites of dye leakage did not coincide with the CK19-positive terminal bile duct epithelium, but instead were predominantly localized within regions adjacent to the PLC structures".
We thank the reviewer for the valuable comment. We have added the corresponding quantitative analysis to support this conclusion. Quantitative assessment of the extended-depth imaging data revealed that dye leakage predominantly occurred in regions adjacent to the PLC structure, rather than in the perivenous sinusoidal areas. The corresponding results have been presented in the revised Figure 3G.
(6) Similarly, Figure 4F is central to the Sca1CD34 cell type identification but lacks any quantification, providing it would strengthen the key statement of the article. A possible way to approach this is also by FACS sorting the double-positive cells and bluk/qRT validation.
We thank the reviewer for raising this point. We agree that quantitative validation of the Sca1⁺CD34⁺ population by FACS sorting could further support our conclusions. However, the primary focus of this study is on the spatial localization and transcriptional features of PLC endothelial cells. The identification of the Sca1⁺CD34⁺ subset is robustly supported by multiple complementary approaches, including three-dimensional imaging, co-staining with pan-endothelial markers, and projection mapping analyses. Collectively, these lines of evidence provide a solid basis for characterizing this unique endothelial population.
(7) The images in Figure S4D are not comparable, as the Sca1-stained image shows a longitudinal section of the PV, but the other stainings are cross-sections of PVs.
We thank the reviewer for the careful comment. We agree that the original Sca1-stained image, being a longitudinal section of the portal vein, was not optimal for direct comparison with other cross-sectional images. We have replaced it with a cross-sectional image of the portal vein to ensure comparability across all images. The updated image has been included in the revised Supplementary Figure S4D.
(8) I might be wrong, but Figure 4J is entirely missing, and only a cartoon is provided. Either remove the results part or provide the data.
We appreciate the reviewer’s careful observation. Figure 4J was intentionally designed as a schematic illustration to summarize the structural relationships and spatial organization of the portal vein, hepatic artery, and PLC identified in the previous panels (Figures 4A–4I). It does not represent newly acquired experimental data, but rather serves to provide a conceptual overview of the findings.
To avoid misunderstanding, we have clarified this point in the figure legend and the main text, stating that Figure 4J is a schematic summary rather than an experimental image. Therefore, we respectfully prefer to retain the schematic figure to aid readers’ interpretation of the preceding results.
(9) The methods section lacks information about the CCL4concentration, and it is thus hard to estimate the dosage of CCL4 received (ml/kg). This is important for the interpretation of the severity of the fibrosis and presence of cirrhosis, as different doses may or may not lead to cirrhosis within the short regimen performed by the authors [PMID: 16015684 DOI: 10.3748/wjg.v11.i27.4167]. Validation of the fibrosis/cirrhosis severity is, in this case, crucial for the correct interpretation of the results. If the level of cirrhosis is not confirmed, only progressive fibrosis should be mentioned in the manuscript, as these two terms cannot be used interchangeably.
Thank you for the reviewer’s comment. We indeed omitted the information on the concentration of carbon tetrachloride (CCl<sub>4</sub>) in the Methods section. In our experiments, mice received intraperitoneal injections of CCl<sub>4</sub> at a dose of 1 mL/kg body weight, twice per week, for a total of six weeks. We have revised the manuscript accordingly, using the term “progressive fibrosis” to avoid confusion between fibrosis and cirrhosis.
(10) The following statement is not backed by any correlation analysis: "Particularly during liver fibrosis progression, the PLC exhibits dynamic structural extension correlating with fibrosis severity,.. ".
We thank the reviewer for the comment. The original statement that the “PLC correlates with fibrosis severity” lacked support from quantitative analysis. To ensure a precise description, we have revised the sentence as follows: “During liver fibrosis progression, the PLC exhibits dynamic structural extension.”
(11) Similarly, the following statement is not followed by data that would address the impact of innervation on liver function: "How the autonomic nervous system regulates liver function in mice despite the apparent absence of substantive nerve fiber invasion into the parenchyma remains unclear.".
This section has been revised. In this study, we analyzed the spatial distribution of nerves in the mouse liver using immunofluorescence staining. The results showed that nerve fibers were almost entirely confined to the portal vein region (Figure S6A). Notably, this distribution pattern differs significantly from that in humans. Previous studies have demonstrated that in the human liver, nerves are not only distributed around the portal vein but also present in the central vein, interlobular septa, and connective tissue of the hepatic parenchyma (Miller et al., 2021; Yi, la Fleur, Fliers & Kalsbeek, 2010).
Previous studies have further explained the physiological basis for this difference: even among species with differences in parenchymal sympathetic innervation (i.e., species with or without direct sympathetic input), their sympathetic efferent regulatory functions may still be similar (Beckh, Fuchs, Ballé & Jungermann, 1990). This is because signals released by adrenergic and peptidergic nerve terminals can be transmitted to hepatocytes as electrical signals through intercellular gap junctions (Hertzberg & Gilula, 1979; Jensen, Alpini & Glaser, 2013; Seseke, Gardemann & Jungermann, 1992; Taher, Farr & Adeli, 2017). However, the scarcity of nerve fibers in the mouse hepatic parenchyma suggests that the mechanism by which the autonomic nervous system regulates liver function in mice may differ from that in humans. This finding also prompts us to further explore the potential role of PLC endothelial cells in this process.
(12) Could the authors discuss their interpretation of the results in light of the fact that the innervation is lower in cirrhotic patients? https://pmc.ncbi.nlm.nih.gov/articles/PMC2871629/. Also, while ADGRG6 (Gpr126) may play important roles in liver Schwann cells, it is likely not through affecting myelination of the nerves, as the liver nerves are not myelinated https://pubmed.ncbi.nlm.nih.gov/2407769/ and https://www.pnas.org/doi/10.1073/pnas.93.23.13280.
We have revised the text to state that although most hepatic nerves are unmyelinated, GPR126 (ADGRG6) may regulate hepatic nerve distribution via non-myelination-dependent mechanisms. Studies have shown that GPR126 exerts both Schwann cell–dependent and –independent functions during peripheral nerve repair, influencing axon guidance, mechanosensation, and ECM remodeling (Mogha et al., 2016; Monk et al., 2011; Paavola et al., 2014).
(13) The manuscript would benefit from text curation that would:
a) Unify the language describing the PLC, so it is clear that (if) it represents protrusions of the portal veins.
We have standardized the description of the PLC throughout the manuscript, clearly specifying its anatomical relationship with the portal vein. Wherever appropriate, we indicate that the PLC represents protrusions associated with the portal vein, avoiding ambiguous or inconsistent statements.
b) Increase the accuracy of the statements.
Examples: "bile ducts, and the central vein in adult mouse livers."
We have refined all statements for accuracy.
c) Reduce the space given to discussion and results in the introduction, moving them to the respective parts. The same applies to the results section, where discussion occurs at more places than in the Discussion part itself.
We have edited the Introduction, removing detailed results and functional explanations, and retaining only a concise overview.
Examples: "The formation of PLC structures in the adventitial layer may participate in local blood flow regulation, maintenance of microenvironmental homeostasis, and vascular-stem cell interactions."
"This finding suggests that PLC endothelial cells not only regulate the periportal microcirculatory blood flow, but also establish a specialized microenvironment that supports periportal hematopoietic regulation, contributing to stem cell recruitment, vascular homeostasis, and tissue repair. "
"Together, these findings suggest the PLC endothelium may act as a key regulator of bile duct branching and fibrotic microenvironment remodeling in liver cirrhosis. " This one in particular would require further validation with protein stainings and similar, directly in your model.
d) Provide a clear reference for the used scRNA seq so it's clear that the data were re-analyzed.
Example: "single-cell transcriptomic analysis revealed significant upregulation of bile duct-related genes in the CD34<sup>+</sup>Sca-1<sup>+</sup> endothelium of PLC in cirrhotic liver, with notably high expression of Lgals1 (Galectin-1) and HGF(Figure 5G) "
When describing the transcriptional analysis of PLC endothelial cells, we explicitly cited the original scRNA-seq dataset (Su et al., 2021), clarifying that these data were reanalyzed rather than newly generated.
e) Introducing references for claims that, in places, are crucial for further interpretation of experiments.
Examples: "It not only guides bile duct branching during development but also"; the authors show no data from liver development.
Thank you for pointing this out. We have revised the relevant statement to ensure that the claim is accurate and well-supported.
f) Results sentence "Instead, bile duct epithelial cells at the terminal ducts extended partially along the canalicular network without directly participating in the formation of the bile duct lumen." Lacks a callout to the respective Figure.
We would like to thank the reviewers for pointing out this issue. In the revised manuscript, the relevant image (Figure 3D) has been clearly annotated with white arrows to indicate the phenomenon of terminal cholangiocytes extending along the bile canaliculi network. Additionally, the schematic diagram on the right side clearly shows the bile canaliculi, cholangiocytes, and bile flow direction using arrows and color coding, thus intuitively corresponding to the textual description.
(14) Formal text suggestions: The manuscript text contains a lot of missed or excessive spaces and several typos that ought to be fixed. A few examples follow:
a) "densely n organized vascular network "
b) "analysis, while offering high spatial "
c) "specific differences, In the human liver, "
d) Figure 4F has a typo in the description.
e) "generation of high signal-to-noise ratio, multi-target " SNR abbreviation was introduced earlier.
f) Canals of Hering, CoH abbreviation comes much later than the first mention of the Canals of Hering.
We thank the reviewer for the helpful comment regarding textual consistency. We have carefully reviewed and revised the entire manuscript to improve the accuracy, clarity, and consistency of the text.