for - health - super food - tepary bean - high protein bean - more than beef - draught resistant crops
- Nov 2025
-
www.youtube.com www.youtube.com
-
- Jul 2025
-
www.youtube.com www.youtube.com
-
he key ingredient that made COVID so unique was a four amino acid sequence inserted into spike protein. So that's 12 nucleotides coding for four amino acids shut down planet earth for a couple of years. That's how powerful this is
for - example - leverage - progress trap - COVID - mix of 4 amino acids inserted into a spike protein
-
- May 2024
-
www.linkedin.com www.linkedin.com
-
launching the first Bezos Center for Sustainable Protein at North Carolina State University
for - fake meat - Bezos Center for Sustainable Protein
-
- Jul 2023
-
www.biorxiv.org www.biorxiv.org
-
Review coordinated by Life Science Editors Foundation
Reviewed by: Dr. Angela Andersen, Life Science Editors Foundation
Potential Conflicts of Interest: None
Punch line: Activation of the yeast AMP-activated protein kinase (AMPK) negatively regulates MAGIC, inhibits the import of misfolded proteins into mitochondria & promotes mitochondrial biogenesis and fitness.
Why is this interesting? Maybe all those healthy things like caloric restriction, intermittent fasting, exercise etc that activate AMPK & extend lifespan do so by inhibiting MAGIC & preventing mitochondrial damage from misfolded proteins.
Background: Metabolic imbalance & loss of proteostasis are interconnected hallmarks of aging and age-related diseases. A mitochondria-mediated proteostasis mechanism called MAGIC (mitochondria as guardian in cytosol) concentrates cytosolic misfolded protein at the surface of mitochondria, where they are disaggregated by molecular chaperones, and then imported for degradation by mitochondrial proteases. Inhibition of this pathway prolongs protein aggregation in cytosol after proteotoxic stress, but excessive misfolded proteins in mitochondria can lead to mitochondrial damage.
Results: • Genetic screen for MAGIC regulators uncovered 145 genes. Loss of Snf1 (AMPK homolog) led to increased mitochondrial import even without proteotoxic stress. In contrast indirect, constitutive activation of Snf1 (e.g. low glucose) prevented the import of misfolded proteins in mitochondria.
• The data suggest that the reduced accumulation of misfolded proteins in mitochondria of Snf1-active cells is not due to enhanced intramitochondrial degradation nor to reduced levels of the misfolded protein, but rather due to blocked mitochondrial import.
• Deletion of HAP4 counteracted Snf1 activation and overexpression of Hap4 alone recapitulated Snf1 activation. The Hap2/3/4/5 complex activates the expression of nuclear encoded mitochondrial proteins. Their data suggest that high expression of mitochondrial preproteins due to an elevated Snf1-Hap4 axis compete with misfolded proteins for mitochondrial import.
• Proteotoxic stress led to a reduced growth rate & reduced mitochondrial fitness in high glucose medium but not under glucose limitation. The data suggest that low glucose, activation of Snf1 & prevention of misfolded protein import into mitochondria prevent the growth defect.
• Many neurodegenerative disease-associated aggregation-prone proteins (α-synuclein, FUSP525L, TDP-43, amyloid beta, C9ORF72-associated poly(GR) dipeptide) are detected in mitochondria of human patients or disease models and impair mitochondrial functions. Their data suggest that the import of α-synuclein & associated reduction in mitochondrial fitness can be counteracted by indirect AMPK/Snf1 activation (i.e. glucose limitation).
• Show data in yeast & human cells.
Discussion: This paper revealed an unexpected link between cellular metabolism and proteostasis through MAGIC/mitochondria.
• Snf1/AMPK is a key regulator of MAGIC & of misfolded protein import into mitochondria.
• Snf1/AMPK balances the mitochondrial metabolic and proteostatic functions in response to glucose availability and protects mitochondrial fitness under proteotoxic stress.
• The authors speculate that in high glucose, cells rely on glycolysis for ATP production and mitochondria ‘moonlighting’ in cellular proteostasis through MAGIC, but when glucose is limited and cells rely on oxidative phosphorylation for ATP generation, AMPK is activated and shuts down MAGIC, prioritizing the import of essential mitochondrial preproteins to ensure mitochondrial fitness and energy production.
• Acknowledge limitations: Snf1/Hap4 activation elevates the expression of hundreds of mitochondrial preproteins, not clear whether specific preproteins or cytosolic factors directly involved in inhibiting mitochondrial import, & that more details on mechanisms will be of interest.
• Caloric restriction & AMPK activation might contribute to lifespan extension by inhibiting MAGIC. In human, AMPK activity is elevated during health-benefitting activities such as exercise. Their data suggest that elevating AMPK activity may be beneficial in alleviating proteotoxicity associated with degenerative diseases - but hyperactivated AMPK has also been reported in several neurodegenerative diseases with proteostasis decline (Ang wonders- maybe AMPK is overwhelmed?).
THIS IS A GORGEOUS PAPER!
Future work - I can't wait to see the characterization of the ribosome biogenesis genes that they also pulled out as MAGIC regulators. Anticipating a translation, misfolded protein, mitochondria, aging axis :)
-
-
10.42.28.131:5000 10.42.28.131:5000
-
Trk
-
Flt
-
Tie
-
Abl
protein
-
- May 2023
-
www.youtube.com www.youtube.com
-
examples of high protein (hard) wheats: - Khorasan - Durum - Hard White Wheat - Hard Red Wheat - Red Fife
Good for breads, pizza, and things where chew is more valuable.
-
examples of lower protein (soft) wheats: - Sonora - Spelt - Soft White - Soft Red - Einkorn
good for pastries, cakes, cookies, etc.
-
- Mar 2023
-
-
Review coordinated by Life Science Editors Foundation
Reviewed by: Dr. Angela Andersen
Potential Conflicts of Interest: None
Background: * mRNAs in polarized cells often have a distinct spatial localization patterns that enable localized protein production * In non-polarized cells, mRNAs encoding membrane and secretory proteins are predominantly translated on the endoplasmic reticulum (ER), some mRNAs are enriched on the mitochondrial surface, some mRNAs are bound to the RNA-binding protein (RBP) TIS11B at the surface of the rough ER in "TIS granules". * The translation of specific mRNAs in TIS granules allows assembly of protein complexes that cannot be established when the mRNAs are translated on the ER but outside of TIS granules (physiological relevance). * The canonical rough ER (CRER) is distinct from the TIS granule ER (TGER), and both are distinct from the cytosol.
Questions: * Do mRNAs that encode non-membrane proteins differentially localize to the ER or the cytosol? (in steady state) * Does the amount of protein synthesis differ depending on the subcytoplasmic location of an mRNA?
Summary: * A third of mRNAs that encode non-membrane proteins have a biased localization to TGER or CRER, indicating that the ER membrane is a general site of translation for both membrane and non-membrane proteins. * 52% of mRNAs that encode non-membrane proteins have a biased mRNA transcript localization pattern towards a single cytoplasmic compartment. the TGER, CRER or cytosol. * The localization at the TGER or CRER was largely controlled by a combinatorial code of AU-RBPs at the 3'UTR. TIS11B promotes mRNA localization to TGER and TIA1/L1 to CRER. * LARP4B bound to the 3'UTR promotes cytosolic localization. * The location of translation has an independent effect on protein levels independent of the RBPs/3'UTR: redirecting cytosolic mRNAs to the rough ER membrane increased their steady-state protein levels by two-fold, indicating that the ER environment promotes protein expression. * Compartment-enriched mRNAs differed in their mRNA production and degradation rates, as well as functional classes and levels of their encoded proteins. Therefore the cytoplasm is partitioned into different functional and regulatory compartments that are not enclosed by membranes. * low-abundance proteins are translated in the TGER region. mRNAs encoding zinc finger proteins and transcription factors were substantially enriched at the TGER. These gene classes are usually expressed at lower levels than others.. This localization may regulate protein complex assembly (membrane proteins that are translated in the TGER domain establish protein complexes that cannot be formed when the proteins are translated on the CRER). The TGER may ensure that low-abundance mRNAs are effectively translated into low-abundance proteins. * mRNAs that are the most stable and encode the most highly expressed proteins are enriched on the CRER and include helicases, cytoskeleton-bound proteins, and chromatin regulators, overturning the idea that most non-membrane protein-encoding mRNAs are translated in the cytosol. * mRNAs overrepresented in the cytosol had the highest production and degradation rates and were enriched in proteins involved in mRNA processing and translation factors, whose abundance levels require tight control.
Advance: Evidence for functional compartmentalization of non-membrane mRNA protein expression in the cytosol vs ER. In steady state, general localization of mRNAs to the ER promotes high protein levels.
Significance: Engineered 3'UTR sequences could potentially boost protein expression by localizing mRNAs to the ER in experimental settings, for vaccines etc.
Remaining questions/points: * How does the rough ER stimulate protein expression? * Does the mRNA localization affect complex formation and/or function of non-membrane proteins? * Does this occur in cells other than HEK293T? * Is this regulated?
-
- Dec 2022
-
bio.libretexts.org bio.libretexts.org
-
nuclear pore complex.
Nuclear pore complex or NPC. There is a size exclusion. <60kDa to pass through unassisted.
-
The SRP is a G-protein and exchanges its bound GDP for a GTP upon binding to a protein’s signal sequence.
As soon as this sequence is translated, the SRP binds it. Elongation is temporarily arrested. SRP-ribosome complex binds to the SRP receptor on the ER membrane. Translation now continues into the lumen of the ER. The SPR and its receptor are recycled.
-
-
bio.libretexts.org bio.libretexts.org
-
a stop-transfer sequence (a hydrophobic domain within the polypeptide chain) traps the protein in the fatty acid interior
-
- Oct 2022
-
bio.libretexts.org bio.libretexts.org
-
Each variable group on an amino acid gives that amino acid specific chemical properties (acidic, basic, polar, or nonpolar). This gives each amino acid R group different chemical properties
What gives Amino acids their specific special properties?
-
The carboxyl group of one amino acid and the amino group of the incoming amino acid combine, releasing a molecule of water and creating the peptide bond.
How are peptide bonds formed? What is released?
-
Each amino acid is attached to another amino acid by a covalent bond, known as a peptide bond,
What is a peptide bond?
-
There are 20 genetically encoded amino acids available to the cell to build in proteins and all of these contain the same core sequence: N-C-C- where the first ("alpha") C will always carry the R group and the second will have a double (ketone) bond to oxygen
What is the core sequence to a protein structure? Which letters are the Terminus and which is the alpha C?
-
Each amino acid has the same core structure, which consists of a central carbon atom, also known as the alpha (α) carbon, bonded to an amino group (NH2), a carboxyl group (COOH), and a hydrogen atom.
What is in a protein structure
-
Amino acids are the monomers that make up proteins.
What are Amino Acids?
-
- Aug 2022
-
www.medrxiv.org www.medrxiv.org
-
Thuluva, S., Paradkar, V., Turaga, K., Gunneri, S., Yerroju, V., Mogulla, R., Kyasani, M., Manoharan, S. K., Medigeshi, G., Singh, J., Shaman, H., Singh, C., & Rao, A. V. (2022). Selection of optimum formulation of RBD-based protein sub-unit covid19 vaccine (Corbevax) based on safety and immunogenicity in an open-label, randomized Phase-1 and 2 clinical studies (p. 2022.03.08.22271822). medRxiv. https://doi.org/10.1101/2022.03.08.22271822
-
-
www.livemint.com www.livemint.com
-
Banerjee, P. (2022, February 13). Biological E seeks EUA for its Corbevax vaccine for 12-18 yrs age group. Mint. https://www.livemint.com/news/india/biological-e-seeks-eua-for-its-corbevax-vaccine-for-12-18-yrs-age-group-11644746753338.html
-
-
elifesciences.org elifesciences.org
-
Li, Z., Tomlinson, A. C., Wong, A. H., Zhou, D., Desforges, M., Talbot, P. J., Benlekbir, S., Rubinstein, J. L., & Rini, J. M. (2019). The human coronavirus HCoV-229E S-protein structure and receptor binding. ELife, 8, e51230. https://doi.org/10.7554/eLife.51230
-
-
twitter.com twitter.com
-
nature. (2021, November 9). Protein-based vaccines—With their inexpensive production protocols and logistical advantages—Could help to narrow the immunization gap between rich and poor countries https://t.co/pLunUiQl3n [Tweet]. @Nature. https://twitter.com/Nature/status/1458009972214747136
-
- Apr 2022
-
twitter.com twitter.com
-
nature. (2021, April 16). Coronavirus variants: Where do they come from? How do we spot them? What do they mean for COVID vaccines, and future of the pandemic? Https://t.co/NRbORu2hoF [Tweet]. @Nature. https://twitter.com/Nature/status/1383093697374474240
-
- Mar 2022
-
www.forbes.com www.forbes.com
-
Haseltine, W. A. (n.d.). Birth Of The Omicron Family: BA.1, BA.2, BA.3. Each As Different As Alpha Is From Delta. Forbes. Retrieved 30 March 2022, from https://www.forbes.com/sites/williamhaseltine/2022/01/26/birth-of-the-omicron-family-ba1-ba2-ba3-each-as-different-as-alpha-is-from-delta/
-
-
www.news18.com www.news18.com
-
Corbevax: All About India’s First Protein Sub-Unit Covid Vaccine to be Given to 12-14 Age Group From Today. (2022, March 16). News18. https://www.news18.com/news/india/corbevax-all-about-indias-first-protein-sub-unit-covid-vaccine-to-be-given-to-12-14-age-group-from-today-4874345.html
-
-
www.bmj.com www.bmj.com
-
Mahase, E. (2022). Covid-19: Sanofi and GSK to seek regulatory authorisation for protein based vaccine. BMJ, 376, o526. https://doi.org/10.1136/bmj.o526
-
- Feb 2022
-
twitter.com twitter.com
-
Trevor Bedford. (2022, January 28). Omicron viruses can be divided into two major groups, referred to as PANGO lineages BA.1 and BA.2 or @nextstrain clades 21K and 21L. The vast majority of globally sequenced Omicron have been 21K (~630k) compared a small minority of 21L (~18k), but 21L is gaining ground. 1/15 [Tweet]. @trvrb. https://twitter.com/trvrb/status/1487105396879679488
-
-
twitter.com twitter.com
-
Prof. Akiko Iwasaki. (2022, January 27). Vaccines that reduce infection & disease are needed to combat the pandemic. Here, @tianyangmao @BenIsraelow et al. Describe our new mucosal booster strategy, Prime and Spike, to induce such immunity via nasal delivery of unadjuvanted spike vaccine 🧵 (1/) https://biorxiv.org/content/10.1101/2022.01.24.477597v1 https://t.co/bcB5MFph9F [Tweet]. @VirusesImmunity. https://twitter.com/VirusesImmunity/status/1486510697332842498
-
-
www.theguardian.com www.theguardian.com
-
Devlin, H., & correspondent, H. D. S. (2022, February 3). Novavax Covid vaccine approved for use in over-18s in UK. The Guardian. https://www.theguardian.com/society/2022/feb/03/novavax-covid-vaccine-approved-for-use-in-over-18s-in-uk
-
-
www.statnews.com www.statnews.com
-
Moderna wins full approval for its Covid-19 vaccine, as Novavax seeks authorization for its version. (2022, January 31). STAT. https://www.statnews.com/2022/01/31/and-then-there-were-2-fda-gives-full-approval-to-modernas-covid-19-vaccine/
-
- Jan 2022
-
retractionwatch.com retractionwatch.com
-
Marcus, A. A. (2022, January 13). COVID-19 spike protein paper earns an expression of concern. Retraction Watch. https://retractionwatch.com/2022/01/13/covid-19-spike-protein-paper-earns-an-expression-of-concern/
-
- Dec 2021
-
twitter.com twitter.com
-
Eric Topol. (2021, November 27). View of key mutations of Omicron’s 50, with 30 in the spike protein, 15 in its receptor binding domain https://ft.com/content/42c5ff3d-e676-4076-9b9f-7243a00cba5e http://covariants.org @EllingUlrich @_b_meyer https://t.co/onMoNFVLFJ [Tweet]. @EricTopol. https://twitter.com/EricTopol/status/1464452102382448643
-
-
ir.novavax.com ir.novavax.com
-
Novavax and Serum Institute of India Announce World Health Organization Grants Emergency Use Listing for NVX-CoV2373 COVID-19 Vaccine—Dec 17, 2021. (n.d.). Retrieved December 18, 2021, from https://ir.novavax.com/2021-12-17-Novavax-and-Serum-Institute-of-India-Announce-World-Health-Organization-Grants-Emergency-Use-Listing-for-NVX-CoV2373-COVID-19-Vaccine?sf156899079=1
Tags
- India
- COVID-19
- lang:en
- protein-based vaccine
- Novavax
- WHO
- emergency
- vaccine
- emergency use listing
- is:webpage
Annotators
URL
-
-
www.gavi.org www.gavi.org
-
What do we know about the new B.1.1.529 coronavirus variant and should we be worried? (n.d.). Retrieved 10 December 2021, from https://www.gavi.org/vaccineswork/what-we-know-about-new-b11529-coronavirus-variant-so-far
-
-
www.bbc.co.uk www.bbc.co.uk
-
New Covid variant: How worried should we be? (2021, November 25). BBC News. https://www.bbc.com/news/health-59418127
-
-
www.theguardian.com www.theguardian.com
-
Devlin, H., & Kollewe, J. (2021, November 26). BioNTech says it could tweak Covid vaccine in 100 days if needed. The Guardian. https://www.theguardian.com/society/2021/nov/26/biontech-says-it-could-tweak-covid-vaccine-in-100-days-if-needed
Tags
- Pfizer
- science
- transmissibility
- Omicron
- lang:en
- protection
- immunity
- escape variant
- vaccine
- development
- clinical trials
- research
- booster
- variant
- BioNTech
- mutation
- is:news
- COVID-19
- variant-specific booster
- effectiveness
- spike protein
Annotators
URL
theguardian.com/society/2021/nov/26/biontech-says-it-could-tweak-covid-vaccine-in-100-days-if-needed -
-
www.abbott.com www.abbott.com
-
Evaluating Omicron and Other COVID Variants to Ensure Test Effectiveness. (n.d.). Abbott. Retrieved December 3, 2021, from https://www.abbott.com/corpnewsroom/diagnostics-testing/monitoring-covid-variants-to-ensure-test-effectiveness.html
-
- Nov 2021
-
www.nature.com www.nature.com
-
Those structures reveal that Ag+ binds to 6PGDH at both catalytic and non-catalytic sites with dominant binding residues of Cys, His and Met in quasi-linear and trigonal geometry, which is generally consistent with our previous reports36,40. Together with the site-directed mutagenesis study, we unveil that Ag+ abolishes the enzymatic activity of 6PGDH through targeting His185 in the active site and morphing its catalytic pocket.
Ag can bind with protein at Cys, His and Met residues.
-
- Oct 2021
-
www.nature.com www.nature.com
-
Sun, W., Liu, Y., Amanat, F., González-Domínguez, I., McCroskery, S., Slamanig, S., Coughlan, L., Rosado, V., Lemus, N., Jangra, S., Rathnasinghe, R., Schotsaert, M., Martinez, J. L., Sano, K., Mena, I., Innis, B. L., Wirachwong, P., Thai, D. H., Oliveira, R. D. N., … Palese, P. (2021). A Newcastle disease virus expressing a stabilized spike protein of SARS-CoV-2 induces protective immune responses. Nature Communications, 12(1), 6197. https://doi.org/10.1038/s41467-021-26499-y
-
- Aug 2021
-
-
Greinacher, A., Thiele, T., Warkentin, T. E., Weisser, K., Kyrle, P. A., & Eichinger, S. (2021). Thrombotic Thrombocytopenia after ChAdOx1 nCov-19 Vaccination. New England Journal of Medicine, 384(22), 2092–2101. https://doi.org/10.1056/NEJMoa2104840
-
-
www.thelancet.com www.thelancet.com
-
Shrotri, M., Navaratnam, A. M. D., Nguyen, V., Byrne, T., Geismar, C., Fragaszy, E., Beale, S., Fong, W. L. E., Patel, P., Kovar, J., Hayward, A. C., & Aldridge, R. W. (2021). Spike-antibody waning after second dose of BNT162b2 or ChAdOx1. The Lancet, 398(10298), 385–387. https://doi.org/10.1016/S0140-6736(21)01642-1
-
- Jun 2021
-
www.henryford.com www.henryford.com
-
Here’s Where That COVID-19 Vaccine Infertility Myth Came From—And Why It Is Not True. (n.d.). Retrieved June 18, 2021, from https://www.henryford.com/blog/2021/04/fertility-rumor-covid-vaccine
-
- May 2021
-
www.medrxiv.org www.medrxiv.org
-
Saadat, S., Tehrani, Z. R., Logue, J., Newman, M., Frieman, M. B., Harris, A. D., & Sajadi, M. M. (2021). Single Dose Vaccination in Healthcare Workers Previously Infected with SARS-CoV-2. MedRxiv, 2021.01.30.21250843. https://doi.org/10.1101/2021.01.30.21250843
-
- Apr 2021
-
twitter.com twitter.com
-
Trevor Bedford. (2021, January 14). After ~10 months of relative quiescence we’ve started to see some striking evolution of SARS-CoV-2 with a repeated evolutionary pattern in the SARS-CoV-2 variants of concern emerging from the UK, South Africa and Brazil. 1/19 [Tweet]. @trvrb. https://twitter.com/trvrb/status/1349774271095062528
-
- Mar 2021
-
twitter.com twitter.com
-
DataBeers Brussels. (2020, October 26). ⏰ Our next #databeers #brussels is tomorrow night and we’ve got a few tickets left! Don’t miss out on some important and exciting talks from: 👉 @svscarpino 👉 Juami van Gils 👉 Joris Renkens 👉 Milena Čukić 🎟️ Last tickets here https://t.co/2upYACZ3yS https://t.co/jEzLGvoxQe [Tweet]. @DataBeersBru. https://twitter.com/DataBeersBru/status/1320743318234562561
-
- Feb 2021
-
www.science.org www.science.org
-
Gordon, D. E., Hiatt, J., Bouhaddou, M., Rezelj, V. V., Ulferts, S., Braberg, H., Jureka, A. S., Obernier, K., Guo, J. Z., Batra, J., Kaake, R. M., Weckstein, A. R., Owens, T. W., Gupta, M., Pourmal, S., Titus, E. W., Cakir, M., Soucheray, M., McGregor, M., … Krogan, N. J. (2020). Comparative host-coronavirus protein interaction networks reveal pan-viral disease mechanisms. Science, 370(6521). https://doi.org/10.1126/science.abe9403
-
-
twitter.com twitter.com
-
Andrew💙Croxford. (2020, December 3). NEW THREAD: possible development of anti-Syncytin responses after immunization with the SARS-CoV-2 spike protein-coding mRNA vaccines, based on a ‘homologous’ region shared between these proteins. [Tweet]. @andrew_croxford. https://twitter.com/andrew_croxford/status/1334593606196187136
-
-
www.theguardian.com www.theguardian.com
-
Here’s what we know about the new variant of coronavirus | Sharon Peacock. (2020, December 22). The Guardian. http://www.theguardian.com/commentisfree/2020/dec/22/new-variant-coronavirus-genomic-sars-cov-2-pandemic
-
-
www.medrxiv.org www.medrxiv.org
-
Hodcroft, E. B., Domman, D. B., Oguntuyo, K., Snyder, D. J., Diest, M. V., Densmore, K. H., Schwalm, K. C., Femling, J., Carroll, J. L., Scott, R. S., Whyte, M. M., Edwards, M. D., Hull, N. C., Kevil, C. G., Vanchiere, J. A., Lee, B., Dinwiddie, D. L., Cooper, V. S., & Kamil, J. P. (2021). Emergence in late 2020 of multiple lineages of SARS-CoV-2 Spike protein variants affecting amino acid position 677. MedRxiv, 2021.02.12.21251658. https://doi.org/10.1101/2021.02.12.21251658
-
- Nov 2020
-
www.ncbi.nlm.nih.gov www.ncbi.nlm.nih.gov
-
by attaching acceptor and donor to different domains of a target protein, the interdomain dynamics can be monitored
-
-
www.plymouth.edu www.plymouth.edu
-
proteome
The entirety of proteins that are expressed as a cell, tissue or an organism.
-
-
www.plymouth.edu www.plymouth.edu
-
transcriptionalleve
This is the process of a complementary mRNA copy of a single gene on the DNA that is created in the nucleus. The mRNA is smaller than the DNA so it can carry the genetic code into the ribosome and into the cytoplasm that enables the protein creation.
-
- Oct 2020
-
www.plymouth.edu www.plymouth.edu
-
messenger RNA (mRNA)
This is a single strand on an RNA molecule that leaves the the nucleus of a cell in order to relocate to the cytoplasm. This is where the mRNA can help create the protein for the cell in a process known as protein synthesis. The mRNA takes in information passed into it by DNA and decode it for the ribosomes to make more protein for the cell to live on.
-
- Aug 2020
-
medrxiv.org medrxiv.org
-
Ferretti, A. P., Kula, T., Wang, Y., Nguyen, D. M., Weinheimer, A., Dunlap, G. S., Xu, Q., Nabilsi, N., Perullo, C. R., Cristofaro, A. W., Whitton, H. J., Virbasius, A., Olivier, K. J., Baiamonte, L. B., Alistar, A. T., Whitman, E. D., Bertino, S. A., Chattopadhyay, S., & MacBeath, G. (2020). COVID-19 Patients Form Memory CD8+ T Cells that Recognize a Small Set of Shared Immunodominant Epitopes in SARS-CoV-2. MedRxiv, 2020.07.24.20161653. https://doi.org/10.1101/2020.07.24.20161653
-
-
www.nature.com www.nature.com
-
Corbett, K. S., Edwards, D. K., Leist, S. R., Abiona, O. M., Boyoglu-Barnum, S., Gillespie, R. A., Himansu, S., Schäfer, A., Ziwawo, C. T., DiPiazza, A. T., Dinnon, K. H., Elbashir, S. M., Shaw, C. A., Woods, A., Fritch, E. J., Martinez, D. R., Bock, K. W., Minai, M., Nagata, B. M., … Graham, B. S. (2020). SARS-CoV-2 mRNA vaccine design enabled by prototype pathogen preparedness. Nature, 1–8. https://doi.org/10.1038/s41586-020-2622-0
-
-
-
Walls, A. C., Fiala, B., Schäfer, A., Wrenn, S., Pham, M. N., Murphy, M., Tse, L. V., Shehata, L., O’Connor, M. A., Chen, C., Navarro, M. J., Miranda, M. C., Pettie, D., Ravichandran, R., Kraft, J. C., Ogohara, C., Palser, A., Chalk, S., Lee, E.-C., … King, N. P. (2020). Elicitation of potent neutralizing antibody responses by designed protein nanoparticle vaccines for SARS-CoV-2. BioRxiv, 2020.08.11.247395. https://doi.org/10.1101/2020.08.11.247395
-
-
www.journalofhospitalmedicine.com www.journalofhospitalmedicine.com
-
July 22, J. H. M. 2020 A.-493 P. O. F., & DOI 10.12788/jhm.3497, 2020 |. (2020). Effect of Systemic Glucocorticoids on Mortality or Mechanical Ventilation in Patients With COVID-19. Journal of Hospital Medicine, 15(8). https://doi.org/10.12788/jhm.3497
-
-
www.biorxiv.org www.biorxiv.org
-
Martino, C., Kellman, B. P., Sandoval, D. R., Clausen, T. M., Marotz, C. A., Song, S. J., Wandro, S., Zaramela, L. S., Benítez, R. A. S., Zhu, Q., Armingol, E., Vázquez-Baeza, Y., McDonald, D., Sorrentino, J. T., Taylor, B., Belda-Ferre, P., Liang, C., Zhang, Y., Schifanella, L., … Knight, R. (2020). Bacterial modification of the host glycosaminoglycan heparan sulfate modulates SARS-CoV-2 infectivity. BioRxiv, 2020.08.17.238444. https://doi.org/10.1101/2020.08.17.238444
-
-
www.jpeds.com www.jpeds.com
-
Yonker, L. M., Neilan, A. M., Bartsch, Y., Patel, A. B., Regan, J., Arya, P., Gootkind, E., Park, G., Hardcastle, M., John, A. S., Appleman, L., Chiu, M. L., Fialkowski, A., Flor, D. D. la, Lima, R., Bordt, E. A., Yockey, L. J., D’Avino, P., Fischinger, S., … Fasano, A. (2020). Pediatric SARS-CoV-2: Clinical Presentation, Infectivity, and Immune Responses. The Journal of Pediatrics, 0(0). https://doi.org/10.1016/j.jpeds.2020.08.037
-
-
www.youtube.com www.youtube.com
-
Science Facebook Live: Understanding SARS-CoV-2 structure informs vaccine design, clinical trials. (2020, March 20). https://www.youtube.com/watch?v=nOzEx3OpKe8
-
-
www.biorxiv.org www.biorxiv.org
-
Glasgow, A., Glasgow, J., Limonta, D., Solomon, P., Lui, I., Zhang, Y., Nix, M. A., Rettko, N. J., Lim, S. A., Zha, S., Yamin, R., Kao, K., Rosenberg, O. S., Ravetch, J. V., Wiita, A. P., Leung, K. K., Zhou, X. X., Hobman, T. C., Kortemme, T., & Wells, J. A. (2020). Engineered ACE2 receptor traps potently neutralize SARS-CoV-2. BioRxiv, 2020.07.31.231746. https://doi.org/10.1101/2020.07.31.231746
-
-
-
Bangaru, S., Ozorowski, G., Turner, H. L., Antanasijevic, A., Huang, D., Wang, X., Torres, J. L., Diedrich, J. K., Tian, J.-H., Portnoff, A. D., Patel, N., Massare, M. J., Yates, J. R., Nemazee, D., Paulson, J. C., Glenn, G., Smith, G., & Ward, A. B. (2020). Structural analysis of full-length SARS-CoV-2 spike protein from an advanced vaccine candidate. BioRxiv, 2020.08.06.234674. https://doi.org/10.1101/2020.08.06.234674
-
-
www.youtube.com www.youtube.comYouTube1
-
LucyinLockdown: Viruses, Pandemics and Lessons Learnt with Dr Jane Greatorex—YouTube. (2020, June 18). https://youtu.be/5U1go5hyen8
-
-
www.newscientist.com www.newscientist.com
-
Marshall, M. (n.d.). Everything you need to know about Russia’s coronavirus vaccine claims. New Scientist. Retrieved August 18, 2020, from https://www.newscientist.com/article/2251722-everything-you-need-to-know-about-russias-coronavirus-vaccine-claims/
Tags
- is:news
- bad science
- COVID-19
- lang:en
- transparency
- Russia
- public health
- strategy
- safety
- vaccine
- spike protein
- development
- concern
- efficacy
- immunology
Annotators
URL
-
-
www.biorxiv.org www.biorxiv.org
-
Ou, T., Mou, H., Zhang, L., Ojha, A., Choe, H., & Farzan, M. (2020). Hydroxychloroquine-mediated inhibition of SARS-CoV-2 entry is attenuated by TMPRSS2. BioRxiv, 2020.07.22.216150. https://doi.org/10.1101/2020.07.22.216150
-
-
science.sciencemag.org science.sciencemag.org
-
Hsieh, C.-L., Goldsmith, J. A., Schaub, J. M., DiVenere, A. M., Kuo, H.-C., Javanmardi, K., Le, K. C., Wrapp, D., Lee, A. G., Liu, Y., Chou, C.-W., Byrne, P. O., Hjorth, C. K., Johnson, N. V., Ludes-Meyers, J., Nguyen, A. W., Park, J., Wang, N., Amengor, D., … McLellan, J. S. (2020). Structure-based design of prefusion-stabilized SARS-CoV-2 spikes. Science. https://doi.org/10.1126/science.abd0826
-
-
jamanetwork.com jamanetwork.com
-
Havers, F. P., Reed, C., Lim, T., Montgomery, J. M., Klena, J. D., Hall, A. J., Fry, A. M., Cannon, D. L., Chiang, C.-F., Gibbons, A., Krapiunaya, I., Morales-Betoulle, M., Roguski, K., Rasheed, M. A. U., Freeman, B., Lester, S., Mills, L., Carroll, D. S., Owen, S. M., … Thornburg, N. J. (2020). Seroprevalence of Antibodies to SARS-CoV-2 in 10 Sites in the United States, March 23-May 12, 2020. JAMA Internal Medicine. https://doi.org/10.1001/jamainternmed.2020.4130
-
-
biorxiv.org biorxiv.org
-
Clausen, T. M., Sandoval, D. R., Spliid, C. B., Pihl, J., Painter, C. D., Thacker, B. E., Glass, C. A., Narayanan, A., Majowicz, S. A., Zhang, Y., Torres, J. L., Golden, G. J., Porell, R., Garretson, A. F., Laubach, L., Feldman, J., Yin, X., Pu, Y., Hauser, B., … Esko, J. D. (2020). SARS-CoV-2 Infection Depends on Cellular Heparan Sulfate and ACE2. BioRxiv, 2020.07.14.201616. https://doi.org/10.1101/2020.07.14.201616
-
- Jul 2020
-
www.biorxiv.org www.biorxiv.org
-
Yurkovetskiy, L., Wang, X., Pascal, K. E., Tomkins-Tinch, C., Nyalile, T., Wang, Y., Baum, A., Diehl, W. E., Dauphin, A., Carbone, C., Veinotte, K., Egri, S. B., Schaffner, S. F., Lemieux, J. E., Munro, J., Rafique, A., Barve, A., Sabeti, P. C., Kyratsous, C. A., … Luban, J. (2020). Structural and Functional Analysis of the D614G SARS-CoV-2 Spike Protein Variant. BioRxiv, 2020.07.04.187757. https://doi.org/10.1101/2020.07.04.187757
-
-
www.cell.com www.cell.com
-
How scientists know COVID-19 is way deadlier than the flu. (2020, July 2). Science. https://www.nationalgeographic.com/science/2020/07/coronavirus-deadlier-than-many-believed-infection-fatality-rate-cvd/
-
-
www.biorxiv.org www.biorxiv.org
-
Corbett, K. S., Edwards, D., Leist, S. R., Abiona, O. M., Boyoglu-Barnum, S., Gillespie, R. A., Himansu, S., Schäfer, A., Ziwawo, C. T., DiPiazza, A. T., Dinnon, K. H., Elbashir, S. M., Shaw, C. A., Woods, A., Fritch, E. J., Martinez, D. R., Bock, K. W., Minai, M., Nagata, B. M., … Graham, B. S. (2020). SARS-CoV-2 mRNA Vaccine Development Enabled by Prototype Pathogen Preparedness. BioRxiv, 2020.06.11.145920. https://doi.org/10.1101/2020.06.11.145920
-
-
www.biorxiv.org www.biorxiv.org
-
Zhang, L., Jackson, C. B., Mou, H., Ojha, A., Rangarajan, E. S., Izard, T., Farzan, M., & Choe, H. (2020). The D614G mutation in the SARS-CoV-2 spike protein reduces S1 shedding and increases infectivity. BioRxiv, 2020.06.12.148726. https://doi.org/10.1101/2020.06.12.148726
-
- Jun 2020
-
www.biorxiv.org www.biorxiv.org
-
Starr, T. N., Greaney, A. J., Hilton, S. K., Crawford, K. H., Navarro, M. J., Bowen, J. E., Tortorici, M. A., Walls, A. C., Veesler, D., & Bloom, J. D. (2020). Deep mutational scanning of SARS-CoV-2 receptor binding domain reveals constraints on folding and ACE2 binding [Preprint]. Microbiology. https://doi.org/10.1101/2020.06.17.157982
-
-
twitter.com twitter.com
-
Bloom Lab. (2020, June 18). "We've experimentally measured how all amino-acid mutations to the #SARSCoV2 spike RBD affect ACE2 binding and expression of folded protein in a deep mutational scanning study led by @tylernstarr & Allie Greaney:https://biorxiv.org/content/10.1101/2020.06.17.157982v1 Why is this important? (1/n)" Twitter. https://twitter.com/jbloom_lab/status/1273468539484213248
-
-
www.theatlantic.com www.theatlantic.com
-
Zhang, S. (2020, April 8). The Best Hopes for a Coronavirus Drug. The Atlantic. https://www.theatlantic.com/science/archive/2020/04/what-coronavirus-drug-will-look-like/609661/
-
- May 2020
-
www.nature.com www.nature.com
-
Callaway, E. (2020). The race for coronavirus vaccines: A graphical guide. Nature, 580(7805), 576–577. https://doi.org/10.1038/d41586-020-01221-y
-
-
-
though not conducted in a natural context,
Experiment was performed using purified delftibactin, not whole cell Delftia. While this helps eliminate variables, we must make sure isolation and purification has no effect on protein function.
-
-
www.ncbi.nlm.nih.gov www.ncbi.nlm.nih.gov
-
Wang, C., Li, W., Drabek, D. et al. A human monoclonal antibody blocking SARS-CoV-2 infection. Nat Commun 11, 2251 (2020). https://doi.org/10.1038/s41467-020-16256-y
-
- Jun 2019
-
www.ncbi.nlm.nih.gov www.ncbi.nlm.nih.gov
-
DBP/E4BP4 expression is susceptible to ER stress.
Pay attention. ER Stress, and the Integrated Stress Response as well as Unfolded Protein Response
-
- Apr 2019
-
www.ncbi.nlm.nih.gov www.ncbi.nlm.nih.gov
-
Klotho-deficient mice have accelerated aging phenotypes, whereas overexpression of Klotho in mice extends lifespan. Klotho is an anti-aging single-pass membrane protein predominantly produced in the kidney, with shedding of the amino-terminal extracellular domain into the systemic circulation. Circulating levels of soluble Klotho decrease with age, and the klotho gene is associated with increased risk of age-related diseases. The three forms of Klotho protein have distinct functions. Membrane Klotho forms a complex with fibroblast growth factor (FGF) receptors, functions as an obligatory co-receptor for FGF23, which is involved in aging and the development of chronic diseases via regulation of Pi and vitamin D metabolism. Secreted Klotho functions as a humoral factor with pleiotropic activities including regulation of oxidative stress, growth factor signaling, and ion homeostasis. Secreted Klotho is also involved in organ protection. The intracellular form of Klotho suppresses inflammation-mediated cellular senescence and mineral metabolism. Herein we provide a brief overview of the structure and function and recent research about Klotho.
-
- Jan 2019
-
www.uniprot.org www.uniprot.org
-
-
www.ncbi.nlm.nih.gov www.ncbi.nlm.nih.gov
-
Adipose tissue is no longer considered to be an inert tissue that stores fat. This tissue is capable of expanding to accommodate increased lipids through hypertrophy of existing adipocytes and by initiating differentiation of pre-adipocytes. Adipose tissue metabolism exerts an impact on whole-body metabolism. As an endocrine organ, adipose tissue is responsible for the synthesis and secretion of several hormones. These are active in a range of processes, such as control of nutritional intake (leptin, angiotensin), control of sensitivity to insulin and inflammatory process mediators (tumor necrosis factor α (TNF-α), interleukin-6 (IL-6), resistin, visfatin, adiponectin, among others) and pathways (plasminogen activator inhibitor 1 (PAI-1) and acylation stimulating protein (ASP) for example). This paper reviews some of the biochemical and metabolic aspects of adipose tissue and its relationship to inflammatory disease and insulin resistance.
-
- Jul 2018
-
www.ncbi.nlm.nih.gov www.ncbi.nlm.nih.gov
-
The biochemical changes are impressive.
-
- Apr 2018
-
www.uniprot.org www.uniprot.org
-
AAIEAGRLFL
-
- Jan 2018
-
www.ncbi.nlm.nih.gov www.ncbi.nlm.nih.gov
-
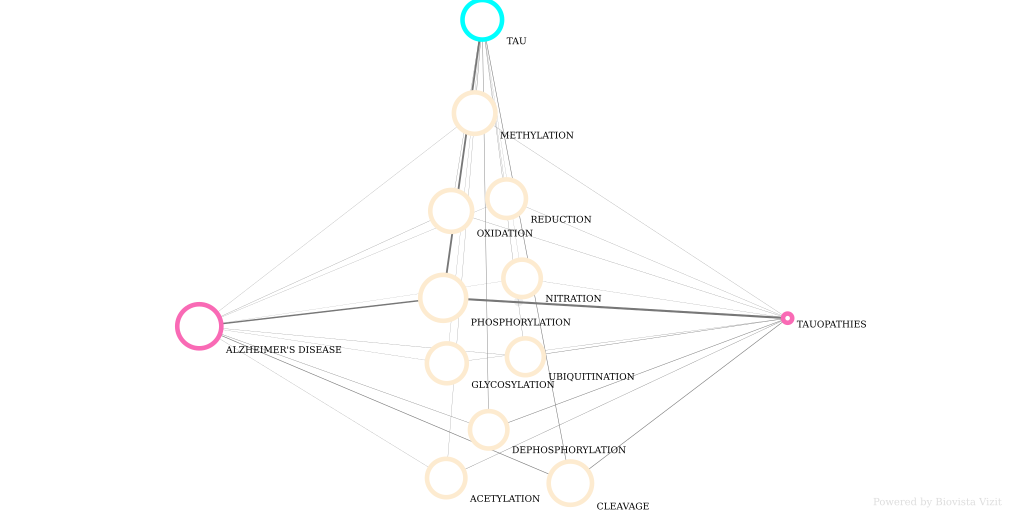
A visual exploration of Tau protein related post-translational modifications in Alzheimers disease (and other tauopathies). Created with Biovista Vizit.
https://www.biovista.com/vizit-research/#!bv_gid=ba502df811e83f68ff3db71d2f33fff3
-
- Feb 2017
-
en.wikipedia.org en.wikipedia.org
- Oct 2016
-
biocontainers.pro biocontainers.pro
-
blast
BLAST finds regions of similarity between biological sequences. The program compares nucleotide or protein sequences to sequence databases and calculates the statistical significance.
-
- May 2015
-
ccdb.ucsd.edu ccdb.ucsd.edu
Tags
Annotators
URL
-